Our Strengths
Global leader in Regenerative Medicine and Cell Therapy
MEDINET offers the specialized technology and expertise required to manage cell processing facilities for regenerative medicine and cell therapy. We provide personnel for cell processing and culturing, quality assurance certification, technology development, and more. With experience in over 190,000 instances of patient-specific cell processing, we offer advanced value-added development and manufacturing expertise to help our clients achieve clinical and commercial success.
Specialist technology and expertise
Delivering regenerative medicine and cell therapy treatments requires numerous processes ranging from cell culturing, freezing and preservation to examination and transport. Ensuring safety and efficiency requires varied, specialized expertise. MEDINET offers the accumulated technology and specialist human resources, as well as the manufacturing management, quality assurance, and personnel management expertise to flexibly meet client needs; from comprehensive programs to processes combinations matching specific requirements.
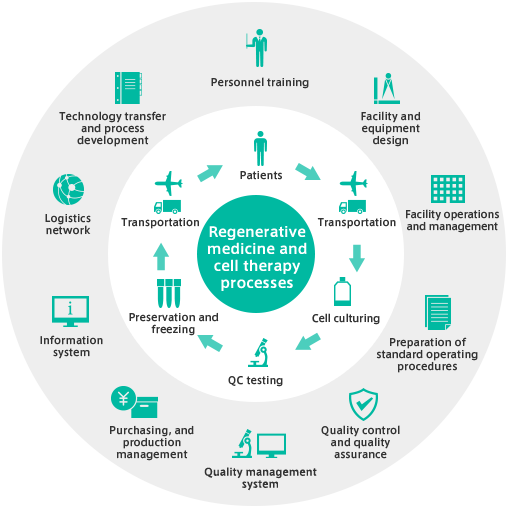
Expertise based on a solid track record
● Experience in over 190,000 instances of patient-specific cell processing
● Medical institutions using MEDINET’s technologies and service
- National Hospital Organization Osaka Medical Center
- Kanazawa University
- Center for Advanced Medical Innovation, Kyushu University
- The Medical Corporation KOSHIKAI, Seta Clinic Group
- 22nd Century Medical and Research Center, University of Tokyo Hospital, etc.
Facility
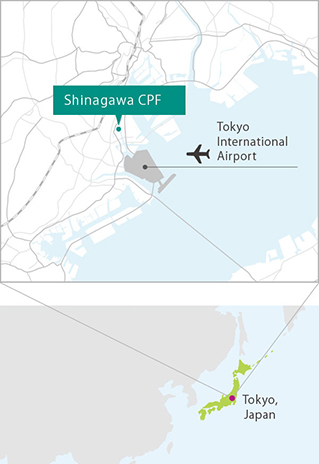
Regenerative medicine and cell therapy require specialized facility and expert personnel. Our Shinagawa Cell Processing Facility (CPF) is the most advanced of its kind in Japan. MEDINET is playing a key role in supporting the client’s development and commercialization of regenerative medicine and cell therapy rapidly, with high levels of safety.
Cell processing facility designed to enable compliance with Japanese, US, and European regulations
Trusted safety management and reliability assurance systems
The Shinagawa CPF's culturing management and quality assurance systems that build on our expertise and MHLW standards. To ensure the independence of cell processing from inspection, MEDINET's assurance incorporates clearly operational responsibilities.

Proximity to Tokyo International Airport provides ready transportation
The Shinagawa CPF is located near Tokyo International Airport's logistics terminal area. Arrangements with courier companies ensure prompt shipping nationwide.
Our People
MEDINET's competitive advantage in CDMO (Contract Development Manufacturing Organization) is its personnel, who are equipped with extensive experience and skills backed by through training programs. MEDINET can support client development and commercialization of egenerative medicine and cell therapy under one roof.
Cell Processing
MEDINET produces many varieties of cell cultures with the utmost attention to safety and quality by cell processing specialists. With an engineer's mindset and exhaustive training in expert knowledge and techniques, our highly skilled personnel exercise a high level of professional discernment. Supported by our advanced training system, to date more than 190,000 cell cultures have been produced safely and efficiently.
